If you’re striving for a green lawn or vigorously growing plants, soil pH is an important subject to understand. Soil pH is the measure of how alkaline or acidic a soil is, and pH has a large impact on plant health and growth. Sometimes high pH soils are referred to as “sweet” and acidic soils as “sour.”
Most plants will grow well with a soil pH ranging from about 6.0 to 7.0, which is slightly acidic to neutral. Some plants have more specific soil pH requirements. For instance, blueberries require a highly acidic soil, with a pH between 4.5 and 5.5.
Fortunately, there are ways to adjust soil pH. Before you learn about these, it’s important to understand some key details about pH and its influence on plant growth. Understanding soil pH and how it impacts plants can improve success with landscapes, lawns, and gardens.
Soil pH Overview
A pH scale of 0 to 14 is used to show how acidic or alkaline (basic) a soil is. A neutral soil has a pH of 7. Values under 7 indicate increasing soil acidity (lower numbers indicate increasing acidity), while values above 7.0 indicate increasing soil alkalinity (higher numbers indicate increasing alkalinity). Soil pH uses a logarithmic scale, so a single unit of difference indicates a tenfold change. That is, soil at pH of 5.0 has 10 times the amount of acidity as a soil with a pH of 6.0 and 100 times the acidity of a neutral (pH 7.0) soil!
To help understand pH, consider some common substances. Orange juice has a pH of 3.5, meaning it is strongly acidic. Baking soda has a pH of 8.4, meaning it is moderately alkaline.
Soil pH can vary widely from region to region and even within a given region. A soil with a pH of 6.1 to 6.5 is considered slightly acidic, and a soil with a pH of 7.4 to 7.8 is considered slightly alkaline.
Technically, soil pH is a measurement of how many hydrogen (H+) ions are in solution. More acidic soils contain more hydrogen ions.
Nutrient Availability
Soil pH has a strong impact on nutrient availability to plants, and therefore affects plant growth and health. Even if the proper nutrients are applied in fertilizer or present in the soil, if the soil pH is overly high or low, plants will not be able to utilize them.
If you’re noticing the signs of nutrient deficiencies in your lawn or garden, such as yellowing or stunted plants, it’s possible that your soil pH is too high or too low. It is always a good practice to have the soil pH tested before undertaking significant garden or landscaping projects.
The best way to test soil pH is to send a soil sample to a soil testing lab. Many state universities offer soil testing services, and there are also privately-owned labs.
Optimal pH Range for Nutrient Availability
Most plants perform best in soil with a pH of 6.0 to 7.0. At this pH range, there is good availability of nutrients for plant uptake. The image below shows how nutrient availability is affected by soil pH.
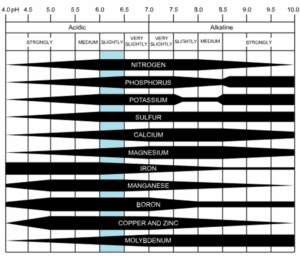
image: CoolKoon
If your soil is overly acidic or too alkaline, certain nutrients will be less available to plants. One of the most commonly seen nutrient deficiencies is lack of iron caused by high soil pH. Note how iron becomes less available at pH above 6.5 in the graphic above. The symptom of iron deficiency is yellow leaf tissue with leaf veins remaining green. This is often called “interveinal chlorosis.” “Chlorosis” is a term for yellowing of plant tissue. “Interveinal” refers to the areas in between leaf veins.
Besides causing nutrient deficiencies due to low nutrient availability and uptake, a pH below 5.5 can also cause nutrient toxicities. As pH decreases, manganese and aluminum become more available. When present and available at high levels, these elements can become toxic to plants.
Its effect on nutrient availability is a key reason that pH is important for healthy plant growth. Fortunately, there are ways to correct a soil’s pH by using certain soil amendments.
Raising Soil pH with Lime
If soil is too acidic, you can increase its pH with lime. Lime is a natural material derived from limestone rock. There are two types of limestone, calcitic limestone and dolomitic limestone. Both contain calcium carbonate (CaCO3), but dolomitic limestone also contains magnesium carbonate (MgCO3). Encap’s Fast Acting™ Lime is made from a very high grade calcitic limestone combined with our Nutri-Bond Technology™.
Just as antacids help indigestion caused by an overabundance of stomach acid, lime application helps to neutralize acidic soils, lowering their pH.
Multiple factors influence how quickly lime reacts to raise pH. One factor is the size of the lime particles, or how finely a lime is ground. Smaller particles react more rapidly than larger particles. Fineness in lime is measured with sieve size ratings, which are listed on the product label. Sieve sizes indicate the percentage by weight of particles that pass through various sized screens. A larger mesh number indicates a finer mesh, so it is desirable to have a high percentage of particles passing through 100-mesh and 200-mesh screens to ensure a fast reaction.
The percentage of calcium carbonate in a lime is another key in its quality. High quality calcitic limes contain at least 90% calcium carbonate.
Earth Science Fast Acting™ Lime uses a very high quality, finely ground calcitic limestone that is at least 92% calcium carbonate and pelletized for easy, dust-free application. The very fine grind of our lime ensures it begins reacting rapidly after application.
Another factor that influences reaction time is the application method, particularly whether it is incorporated into the soil. Lime reacts faster when it is tilled into the soil than when it is broadcast on the soil surface. When applying lime to unplanted areas, it is best to incorporate it into the soil.
Expect to wait a few weeks before lime begins to raise soil pH. If you need to raise your pH by more than one pH point, you may need to apply lime on multiple occasions.
Lowering Soil pH With Sulfur
Elemental sulfur can be used to lower the pH of an alkaline soil. Elemental sulfur is composed one sulfur molecule, rather than sulfur combined with other elements. It’s often referred to as “free sulfur” since it is not bonded to any other elements. Note that elemental sulfur is not soluble and is not available to plants as a food source.
Before applying elemental sulfur, it is helpful to understand how it works. When applied to the soil, microbes convert (oxidize) sulfur to sulfuric acid. The acid reacts to produce sulfate (SO4) and hydrogen ions. It is these hydrogen ions which lower the soil pH. The sulfate produced can be used by plants as a nutrient.
Since elemental sulfur requires on a biological reaction to lower pH, it works best in soils that are moist, warm, and well aerated. These are ideal conditions for microbial life.
Just as with lime, sulfur works faster when incorporated into the soil rather than broadcast on the surface. If a significant soil pH change is desired (over a point of pH change), it is best to apply sulfur a year before planting and incorporate it into the soil well.
An important factor to consider when applying sulfur to lower pH is soil type. Clay soils generally require more sulfur than loamy or sandy soils to lower pH.
Certain types of soil can be resistant to reducing pH with soil amendments. Calcareous soils are soils that are naturally high in limestone, which is composed of calcium carbonate. Soils with high levels of calcium carbonate are resistant to pH reduction because the calcium carbonate present in the soil must be neutralized before the soil pH will change. This can require very large amounts of sulfur, so changing pH of naturally calcareous soils can be challenging and expensive on a large scale. Adding large amounts of organic matter can be helpful in lowering the pH of calcareous soils in smaller yards and gardens.