Soil pH is a measurement of how alkaline (pH > 7.0) or acidic (pH < 7.0) a soil environment is. The pH of a soil has a strong impact on nutrient availability to plants. Most ornamental and vegetable plants prefer a soil pH that is slightly acidic to neutral (6.2 to 7.0) for best growth.
Limestone is a type of sedimentary rock that is rich in calcium carbonate (CaCO3). It is commonly formed when calcium carbonate precipitates in marine deposits over long periods of time, or from the accumulation of debris naturally rich in calcium carbonate (coral, shell, etc.)
There are two types of limestone, calitic lime and dolomitic lime. Calcitic limes contain only low levels of magnesium carbonate (MgCO3), while dolomitic limes contain both magnesium carbonate and calcium carbonate.
Both types of limestone can be used as a soil amendment to raise the pH of acidic soils. Earth Science Fast Acting™ Lime is made of a very finely ground, high quality calcitic lime that is pelletized for easy, dust-free application and combined with our proprietary Nutri-Bond Technology™ to improve its effectiveness.
Lime Quality
A lime’s quality and capability to increase soil pH is based on several factors. For calcitic limestone, the percentage of calcium carbonate the lime contains is a key factor. The best quality calcitic limes contain over 90% calcium carbonate.
Fine grinding also helps to ensure that lime reacts quickly after application, as smaller particles react more quickly in the soil than larger particles. In lime, fineness is measured by sieve size ratings, which are included on the bag’s label. These ratings indicate the percentage by weight of particles passing through various sized screens. The smaller the particles (larger mesh number), the more quickly a lime will react.
Earth Science Fast Acting™ Lime uses a very high quality, finely ground calcitic limestone that is at least 92% calcium carbonate. In terms of fineness, before pelletization 98% of the product passes through a 100-mesh (0.0059 inch or 0.149 mm) screen and 93% through a 200-mesh (0.0029 inch or 0.074 mm) screen. The fine grind of the lime ensures that is begins reacting quickly after application.
Lime as a Soil Amendment
Lime has several benefits when used as a soil amendment. First, it can raise the pH of acidic soils. Although some plants (like azaleas and blueberries) require strongly acidic soil conditions, most plants prefer a pH that is closer to neutral (pH 7.0).
Acidic soils can reduce nutrient availability to plants, including phosphorous. Soil pH also has an important effect on soil microorganisms. Acidic soils generally reduce microbial activity, especially when the soil pH drops below 6.0.
Lime also contains calcium, an essential plant nutrient. Note that it is not the calcium in lime that causes changes in soil pH, but a chemical reaction that converts calcium carbonate to carbon dioxide and water. It is actually the formation of water molecules (using up 2 hydrogen ions from the soil) that reduces soil acidity and increases soil pH.
Our Nutri-Bond Technology™ helps to ensure that applied lime remains in the soil, maximizing its activity. It also stabilizes soil structure, improving water infiltration, reducing runoff and loss of soil and nutrients in runoff water.
Best Practices for Increasing Soil pH with Lime
In areas that have not been planted, incorporate lime into the soil with tillage to speed up its pH adjusting effects. Surface application of lime can be effective, but surface applications take longer to react than sulfur incorporated into the soil.
In areas that require a significant (2 pH points of more) change in soil pH, it is best to begin amending the soil a year before the crop is planted. Larger pH changes (2 pH points or more) take longer than smaller pH changes (1 point or under). Test the soil pH 3 to 4 months after application and re-apply lime if additional pH adjustment is needed.
Other Uses for Lime
Limestone is also used in a range of non-agricultural applications, including “scrubbing” sulfur from industrial emissions to produce gypsum and reduce air pollution.
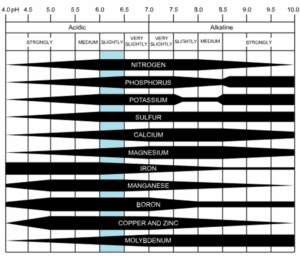
image: CoolKoon